Chemical Kinetics & Reaction Mechanisms
Dr. Thomas Berkemeier, Group Leader
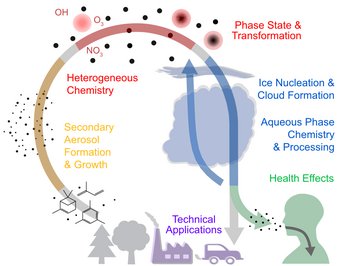
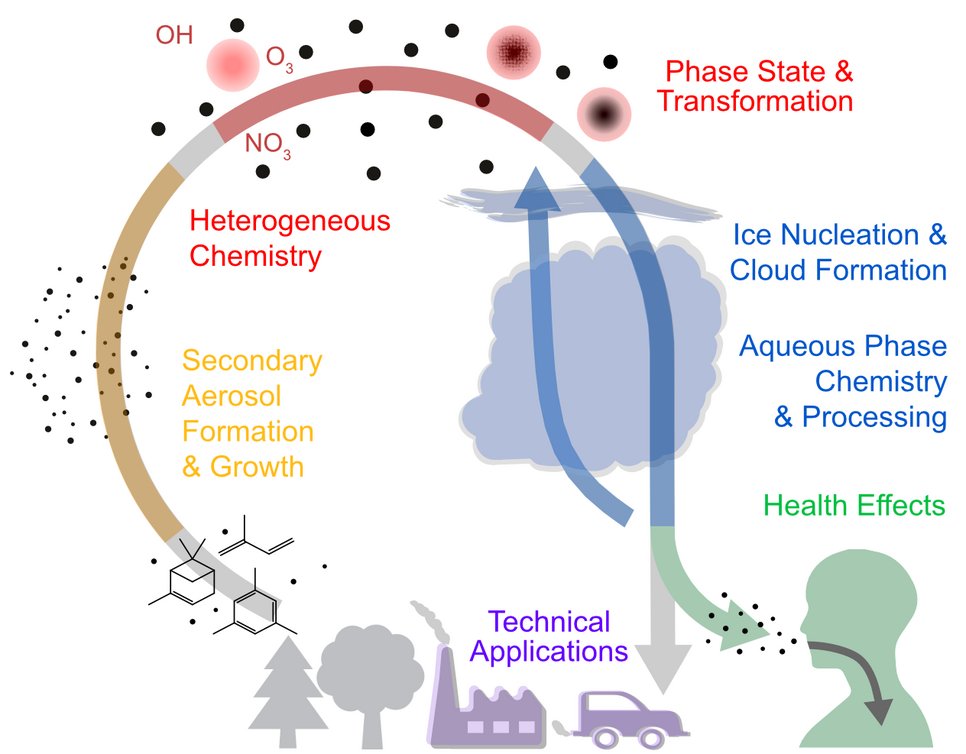
Many chemical processes in the atmosphere, in technical applications, and in the human body occur at and across interfaces and thus constitute multiphase chemical systems. Developing a detailed description of the multiphase reaction kinetics of these systems is vital for understanding the rates at which particles evolve, compounds degrade and oxidants are formed or consumed.
The Berkemeier Group aims at resolving the remaining uncertainties found in complex reaction mechanisms of Atmospheric and Physiological Chemistry, as well as Chemical Technology. For this purpose, we perform laboratory experiments and use elaborate computer models and algorithms that can factor in a large amount of laboratory data.
Key Topics
- Uncovering the underlying mechanisms of air pollution health effects
- Gas-surface interactions (Heterogeneous Chemistry) on atmospheric and technologically-relevant aerosols
- Formation, growth, fate and phase state of secondary organic aerosol (SOA)
- Development of kinetic models, modelling frameworks and optimization algorithms
Methods
- Kinetic multi-layer models
- Kinetic experiments in the laboratory: EPR spectroscopy, chamber and flow reactor studies
- Global optimization methods, sensitivity studies & Machine Learning
Group Members
Thomas Berkemeier (Group Lead)
Anna Backes
Ivan Eremets
Lucia Iezzi
Hyun Gu Kang
Finja Kohl (Student Assistant)
Nina Kropf (Technical Support)
Matteo Krüger
Ashmi Mishra
Maja Radecka
Zhiqiang Zhang