Aerosols, Air Quality & Climate
Yafang Cheng, Minerva Independent Research Group
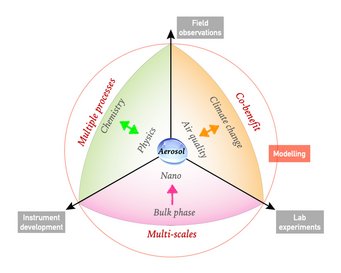
The broad theme of our research is to understand key processes that drive the formation and transformation of aerosols, and to elucidate and quantify the effects of atmospheric aerosols on air quality, public health and climate. The overall goal is to obtain a predictive understanding of the origin, fate and impact of atmospheric aerosols to address the grand challenge of actionable projection of future climate and environment in the Anthropocene.
For this purpose, we combine the development of cutting-edge instruments, lab experiments, field observation and a synthesis approach with model simulations and machine learning techniques that involve multi-scale physical and chemical processes from bulk phase to nanoscale and from regional to global scales.
Current focal points are: (1) developing new pathways and theories of reactive nitrogen chemistry, atmospheric aerosol acidity, and their influence on haze formation (e.g., Science 2011; Science Advances 2016; Science 2020; Chem 2023). (2) improving fundamental understanding of the thermodynamics and molecular dynamics of phase transitions in highly supersaturated nanoparticles and microdroplets, as well as their regional and global effects in the Earth system (e.g., Nature Communications 2015; Science Advances 2018; Chem 2023; Faraday Discuss. 2024). (3) promoting science-oriented policymaking in air pollution control, public health improvement and climate change mitigation, where we demonstrate how a better physico-chemical understanding can help to develop cost-effective control strategies (Science Advances 2016; PNAS 2018; PNAS 2020; Nature Communications 2021; Science 2021; One Earth 2022 & 2023ab, Nature Geoscience 2024).
Group News
- Congratulations to Chaoyang on being awarded the prestigious HORIZON TMA Marie Skłodowska-Curie Actions (MSCA) Postdoctoral Fellowships - European Fellowships (DECCA, Feb 2024).
- Yafang Cheng talked about “Advancing Aerosol Science for the Mitigation of Public Health Risks and Climate Change” at the AAAS Annual Meeting at Denver, USA. (16 Feb 2024)
- Together with Prof. Jintai Lin from Peking University and Junior reserchers Dr. Ruijing Ni, Dr. Chaoqun Ma (from our group) and Dr. Hao Kong (Lin Group), we’re organizing an exciting session “Machine Learning and Other Novel Techniques in Atmospheric and Environmental Science: Application and Development” at the upcoming EGU annual meeting (AS5.5, Vienna, 14-19 April 2024). Welcome to submit abstracts to our session!
- Congradulations to Wenjie and Ruijing on the publication of our study “Ozone pollution mitigation strategy informed by long-term trends of atmospheric oxidation capacity” in Nature Geoscience (Nov 2023).
Research highlights (more)
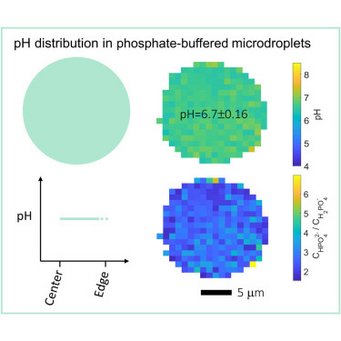
High resolution mapping of aerosol pH in micropdroplets: Li et al., Spatial homogeneity of pH in aerosol microdroplets. Chem (Cell press) 2023.
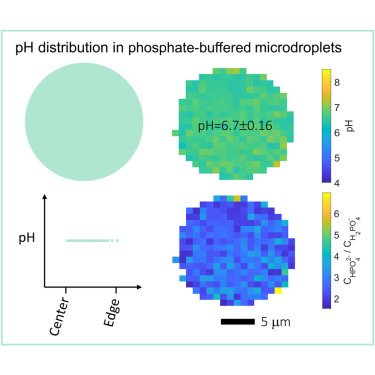
Acidity plays a key role in atmospheric aerosol formation and strongly influences aerosols’ effects on air quality, climate, and the ecosystem. Fundamental questions about aerosol pH are, however, still under debate: how does the pH of aerosol microdroplets compare to that of the parent bulk solution, and do stable pH gradients exist within these microdroplets? Here, by using advanced single-particle and hyperspectral Raman microscopies, we directly measure the pH distribution in NaH2PO4-Na2HPO4 microdroplets (7–10 μm in radius) produced with the conjugate acid-base pair of H2PO4−-HPO42−. We show that the pH values of the investigated microdroplets are indistinguishable from the parent bulk solutions of the same electrolyte concentration and that the pH is essentially constant across the microdroplets, with standard deviations below 0.2 pH units. Similar results are observed for acidic NaHSO4 microdroplets. Our findings are essential to understanding atmospheric multiphase chemistry and overall reactions and processes occurring in aqueous microdroplets.
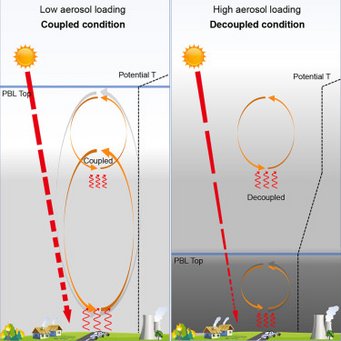
Black carbon aerosols can inhibit the development of the daytime planetary boundary layer (PBL) so significantly that it resembles nighttime conditions: Wang et al., Black-carbon-induced regime transition of boundary layer development strongly amplifies severe haze. One Earth (Cell press) 2023.
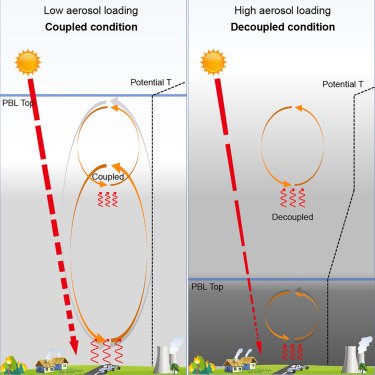
Air pollution is a major threat to human health. Severe haze is often caused by an unexpected extremely shallow planetary boundary layer (PBL), the lowest part of the atmosphere where most pollutants are concentrated. Insufficient understanding of the formation mechanism of a shallow PBL leads to failure of air quality forecast and effective prevention. We found that high atmospheric concentrations of black-carbon (BC) particles (an aerosol) can trigger a “prompt change” in PBL height. Above this tipping point, high concentrations of these particles suppress vertical mixing of atmospheric layers and create a stable PBL, trapping pollutants near the ground and greatly deteriorating air quality. This scenario can be avoided through targeted reductions of BC emissions (rather than targeting total particulate matter reductions). Results further show that mega-wildfires through climate change and nuclear disasters can result in enormous BC emissions and cause extreme stratification of the atmosphere and persistent aerosol layers.
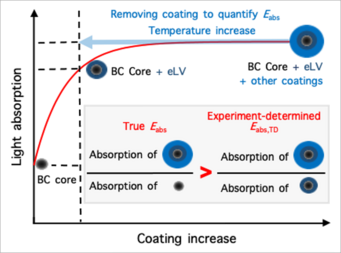
Reconcile debate on black carbon’s climate impact: Zhang et al., Extremely low-volatility organic coating leads to underestimation of black carbon climate impact. One Earth (Cell press) 2023.
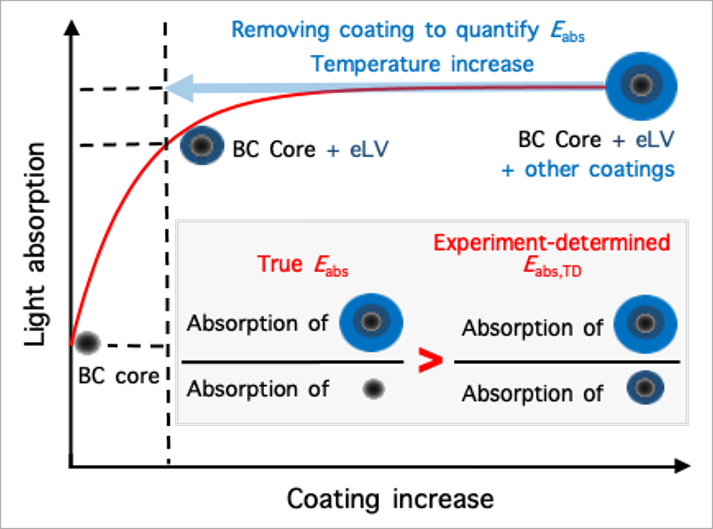
Black carbon (BC) particles come from various incomplete combustions. It can strongly absorb solar radiation and is one of the most important short-lived climate warming agents. In the atmosphere, BC particles become coated via condensation, coagulation, and multiphase reactions. The coatings may greatly enhance BC’s light absorbing efficiency through the so-called “lensing effect,” thereby amplifying its climate warming effect. However, such “lensing effect” was found to be much weaker on BC in ambient air than predicted by theory and lab studies, leading to intensive debate about the actual extent of the coating-induced enhancement of its light absorption and climate impact. We find that this inconsistency arises from the widespread presence of extremely low-volatility (eLV) organics that coat BC. When eLV coatings are quantified and accounted, field observations and theoretical predictions reconcile, showing that coating effects could indeed enhance BC’s climate warming by up to ∼200%.
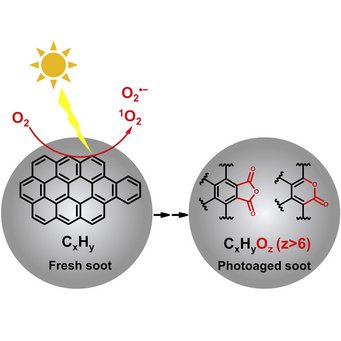
Particle phase chemistry enables soot to better seed clouds: Li et al., Highly oxygenated organic molecules with high unsaturation formed upon photochemical aging of soot. Chem (Cell press) 2022.
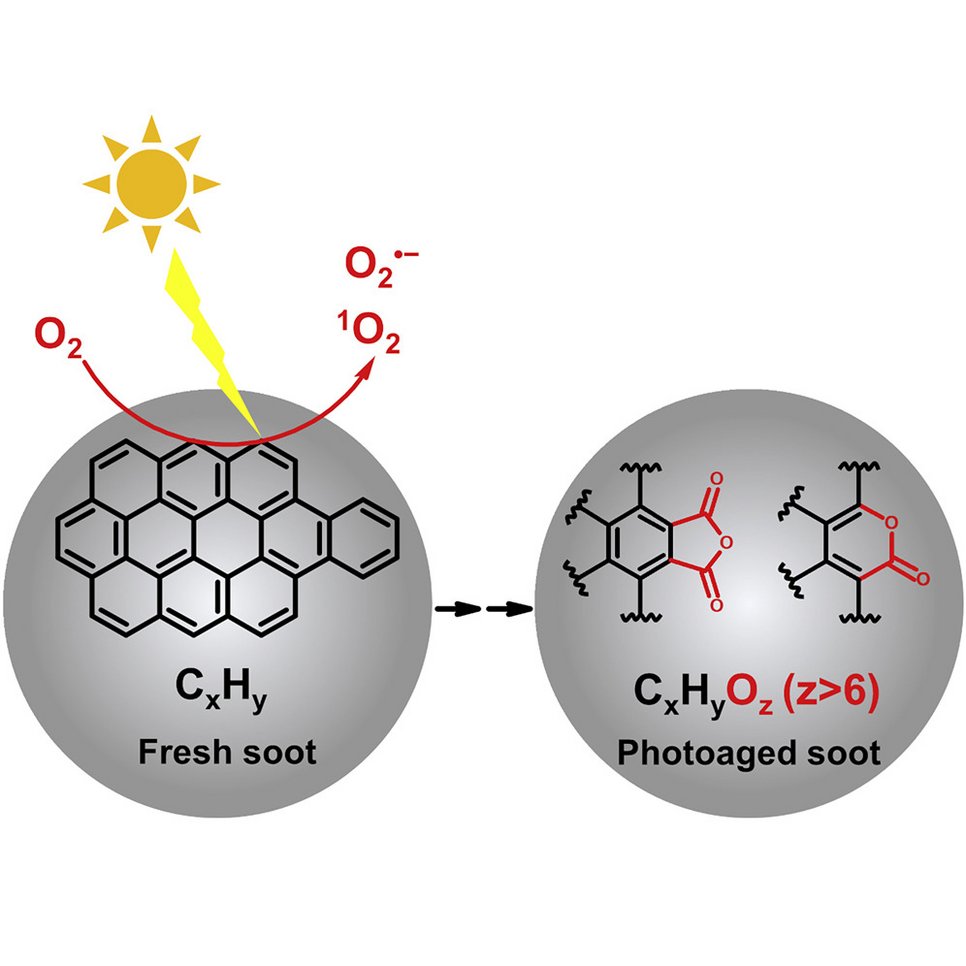
Organic aerosol (OA) is a major component of fine particulate matter in the atmosphere and strongly influences air quality, climate, and human health. However, the formation mechanism of OA is not yet well understood. The oxidation of gas-phase precursors has been considered as the major chemical pathway of OA formation in the atmosphere. We show that particle-phase photochemical oxidation of polycyclic aromatic hydrocarbons on soot can produce a substantial amount of highly oxygenated OA, which changes soot from being hydrophobic to being hydrophilic and increases the ability of soot particles to serve as cloud condensation nuclei. Our finding challenges the current understanding of the formation mechanism of atmospheric secondary organic aerosol and demonstrates a strong impact of particle-phase chemistry on the fate and climate effect of soot and combustion-related organic aerosols (e.g., from wildfires and fossil fuel combustions).
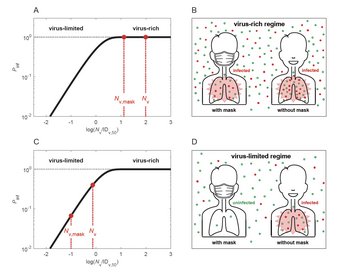
Why and how masks work: Cheng et al., Face masks effectively limit the probability of SARS-CoV-2 transmission. Science 2021.
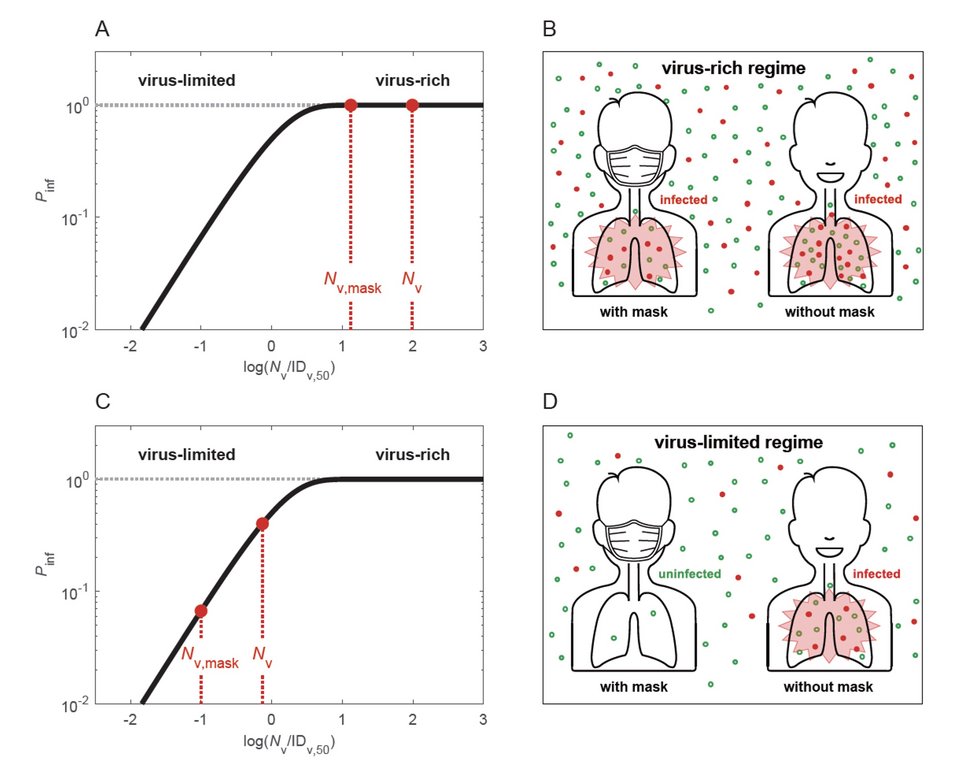
Airborne transmission by droplets and aerosols is important for the spread of viruses. Face masks are a well-established preventive measure, but their effectiveness for mitigating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission is still under debate. We show that variations in mask efficacy can be explained by different regimes of virus abundance and are related to population-average infection probability and reproduction number. For SARS-CoV-2, the viral load of infectious individuals can vary by orders of magnitude. We find that most environments and contacts are under conditions of low virus abundance (virus-limited), where surgical masks are effective at preventing virus spread. More-advanced masks and other protective equipment are required in potentially virus-rich indoor environments, including medical centers and hospitals. Masks are particularly effective in combination with other preventive measures like ventilation and distancing.
Falling Walls Science Breakthroughs of the year 2021 (Physical Sciences) | WoS "hot paper" & "hightly cited paper"|AltMetric Attention Score (>9500), placing it within the top 0.001% of 22 million scientific papers published in total and top 10 of over 77 thousands publications in Science | MPG news | MPIC news | EGU blog | Featured by Bloomberg | Further resources - COVID-19 Aeroosl Transmision Risk Calculator
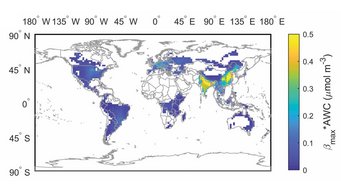
Aerosol pH buffered by ammonia: Zheng et al., Multiphase buffer theory explains contrasts in atmospheric aerosol acidity. Science 2020.
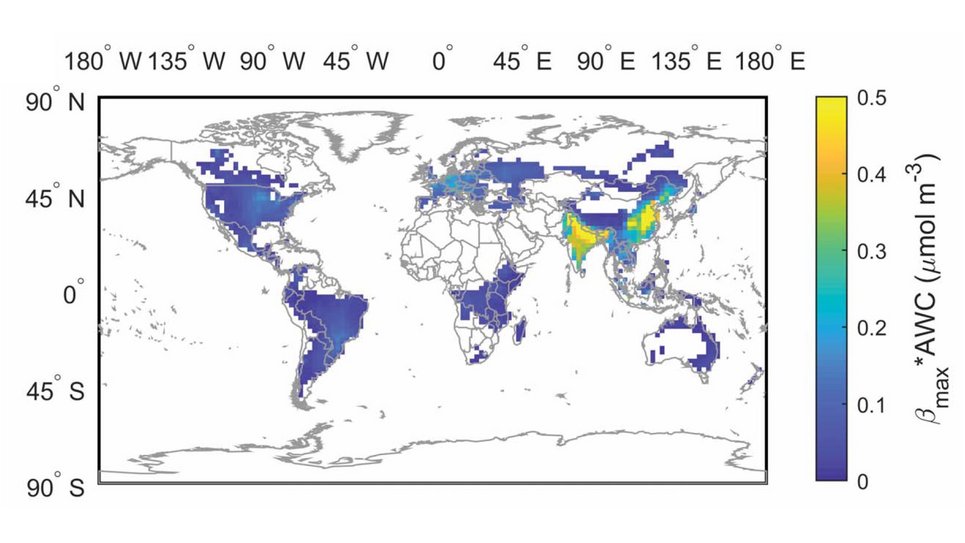
Aerosol acidity largely regulates the chemistry of atmospheric particles, and resolving the drivers of aerosol pH is key to understanding their environmental effects. We find that an individual buffering agent can adopt different buffer pH values in aerosols and that aerosol pH levels in populated continental regions are widely buffered by the conjugate acid-base pair NH4+/NH3 (ammonium/ammonia). We propose a multiphase buffer theory to explain these large shifts of buffer pH, and we show that aerosol water content and mass concentration play a more important role in determining aerosol pH in ammonia-buffered regions than variations in particle chemical composition. Our results imply that aerosol pH and atmospheric multiphase chemistry are strongly affected by the pervasive human influence on ammonia emissions and the nitrogen cycle in the Anthropocene.
MPIC news | WoS "hot paper" and "highly cited paper" | Related studies - Zheng et al, ACP 2022; Zheng et al. ACS Environ. Au 2022
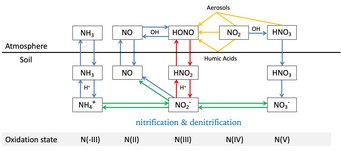
Soil-atmosphere interactions influence atmospheric chemistry: Su and Cheng et al., Soil Nitrite as a Source of Atmospheric HONO and OH Radicals. Science 2011.
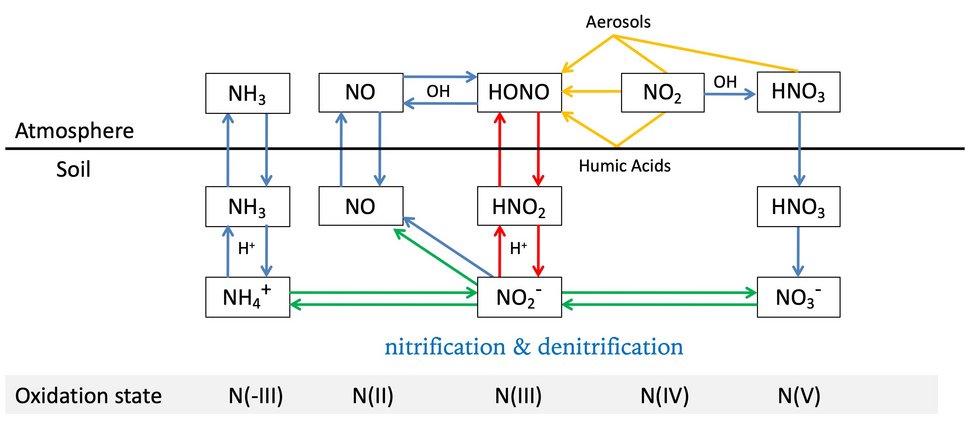
Hydroxyl radicals (OH) are a key species in atmospheric photochemistry. In the lower atmosphere, up to ~30% of the primary OH radical production is attributed to the photolysis of nitrous acid (HONO), and field observations suggest a large missing source of HONO. We show that soil nitrite can release HONO and explain the reported strength and diurnal variation of the missing source. Fertilized soils with low pH appear to be particularly strong sources of HONO and OH. Thus, agricultural activities and land-use changes may strongly influence the oxidizing capacity of the atmosphere. Because of the widespread occurrence of nitrite-producing microbes, the release of HONO from soil may also be important in natural environments, including forests and boreal regions.