Research of the Radical Measurement Group
The goal of the radical measurement group is to investigate radicals, namely hydroxy radicals (OH), hydro peroxy radicals, organic peroxy radicals (RO2) and NO2. The group was established in the Department of Atmospheric Chemistry at the Max Planck Institute for Chemistry in 2002 with a focus on OH- and HO2-radicals.
Since 2005, the instrument we use to measure OH and HO2 in the atmosphere was already deployed on aircrafts, ships and ground stations worldwide, depending on whether we investigate the free and upper troposphere, the marine or the continental boundary layer. Together with complementary measurements of other trace gases and meteorological parameters we are looking for OH and HO2 sources, sinks and their behaviour in different environments.
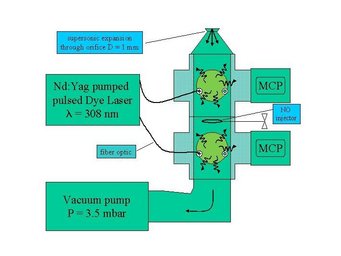
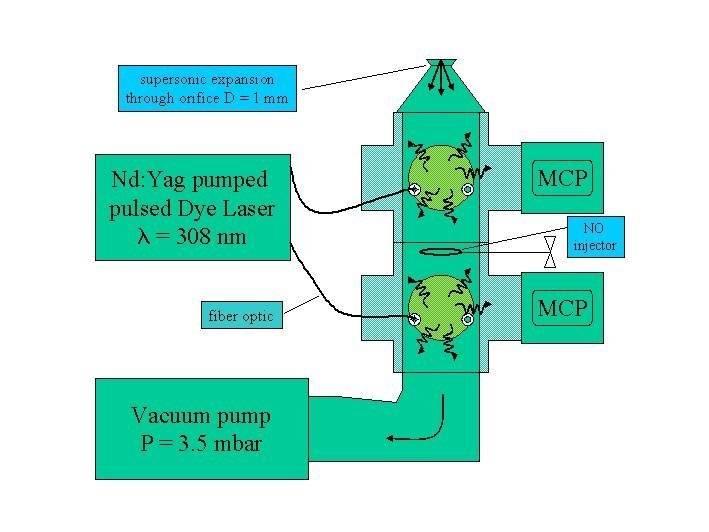
To detect OH, HO2, RO2 and NO2 we use the laser-induced fluorescence (LIF) technique also known as fluorescence assay by gas expansion (FAGE). The air sample is expanded through a pinhole inlet as it is pulled into a reduced pressure detection chamber by a vacuum pump.
The OH molecules in the air are then excited by a laser beam of pulsed light at 308 nm from a frequency doubled dye laser pumped by a Nd:Yag laser. The OH fluorescence extends beyond the prompt scattering on air molecules and particles and is detected with time-gated microchannel plate detectors.
HO2 is measured simultaneously in an additrional detection axis downstream of the OH detection axis through conversion into OH by reaction with NO followed by LIF detection of OH. The NO is added through a loop in between the axes. Calibration is accomplished by photolysis of a known amount of water vapour, producing equal amounts of OH and HO2.
The RO2 instrument is a stand-alone system, which can also be integrated into the OH and HO2 instrument. To detect RO2 it is chemically converted into OH. Therefore, air is drawn into a low pressure reactor where NO and CO are added to the air sample. RO2 reacts with the NO yielding in HO2 (and NO2), which again is converted into OH through the reaction with NO. To extend the lifetime of OH virtually it is back-cycled into HO2 by the reaction of OH with CO. A sample of the “reactor” air is pulled into another low pressure system where NO is added to quantitatively convert HO2 into OH which is subsequently detected.
The NO2 instrument can also be used as a stand-alone instrument or can be integrated into the OH and HO2 instrument. If integrated into the HOx system the NO2 detection axis is mounted between the OH and HO2 detection axis. Light of the wavelength at about 449 nm is used to excite the NO2 molecules and the resulting fluorescence is observed.