Competition in the lungs
Particulate matter overrides the breakdown of hydrogen peroxide. This can lead to adverse health effects from oxidative stress.
According to epidemiological studies, air pollution remains one of the greatest health risks worldwide. The World Health Organization (WHO) has therefore recently tightened their recommendations for acceptable air pollution. The chemical processes underlying the adverse health effects of air pollution, however, are still largely unclear. A deeper understanding of these processes is necessary in order to make further recommendations to curb air pollution and thus effectively reduce disease and mortality.

Together with colleagues at the University of California at Irvine, researchers at the Max Planck Institute for Chemistry (MPIC) have simulated the chemical reactions that occur when particulate matter, ozone, and nitrogen dioxide are inhaled. These air pollutants are most strongly associated with disease and are the main components of urban air pollution. The international team used computer models to recreate the chemical processes in the epithelial fluid, a thin aqueous layer that protects our lungs.
“When exposed to pollutants, the defense mechanisms in our body can become overwhelmed, resulting in the formation of hydroxyl radicals that trigger oxidative stress. For our study, we recreated these processes in the lungs,” explains Thomas Berkemeier, head of the Chemical Kinetics & Reaction Mechanisms group at the MPIC. “We found that even low concentrations of particulate matter can cause the body’s defenses to be bypassed.” For respirable particles smaller than 2.5 micrometer (PM2.5), the model demonstrated clear effects – even at comparatively low pollution levels outside city centers. For Berkemeier, the new WHO guidelines are thus consequential. The recommended value for PM2.5 had been halved from 10 to 5 microgram per cubic meter air end of September.
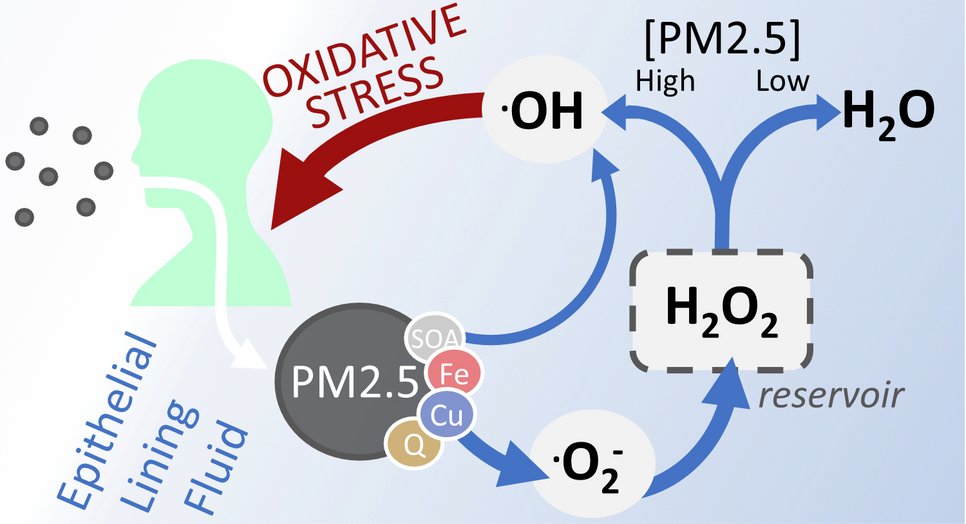
Defense mechanisms already bypassed at low pollution levels
In the recently published study, the molecule hydrogen peroxide (H2O2) is at the center of the chemical mechanism. It can be formed and accumulate in the lungs in small quantities. If we breathe only clean air, enzymes convert most of the hydrogen peroxide into harmless molecules such as water. However, pollutants compete with the enzymes and transform the hydrogen peroxide into highly reactive hydroxyl radicals (OH), which damage biomolecules such as proteins and lipids in the lungs, thereby causing oxidative stress. Depending on its origin, particulate matter may contain copper and iron ions that promote the conversion of hydrogen peroxide into hydroxyl radicals. Chemists call this the Fenton reaction.
“Because they are so reactive, hydroxyl radicals cannot be effectively scavenged by antioxidants. The only protection against these radicals is to prevent them from being formed in our body,” says Steven Lelieveld, a member of the research group and lead author of the study. “This can only be achieved when the air we breathe is as clean as possible.”
“This study is a major step towards a better understanding of the adverse health effects caused by air pollution. The new model is a quantitative tool to evaluate the effects and interactions of different air pollutants,” says Ulrich Pöschl, Head of the Department of Multiphase Chemistry at the MPIC. “Air pollution is still one of the leading causes of excess mortality worldwide. We will link the newly gained knowledge with epidemiological data to give recommendations for effective air pollution control strategies.”