Catalyzer for clean rainforest air
The plant odor isoprene buffers the amount of air-cleaning hydroxyl radicals
Billions of tons of natural and anthropogenic gases are emitted into the Earth's atmosphere every year. If these gases would not be removed by chemical reactions, global warming would be even larger. The most important cleansing agent of the atmosphere is the hydroxyl radical (OH) which catalyzes the oxidation of volatile organic compounds such as methane or isoprene. The reaction with the short-lived but highly reactive molecule transforms these gases into water soluble compounds that can be removed from the atmosphere by precipitation. The oxidation also leads the formation of ozone and aerosol particles, which in turn affect air quality and climate.
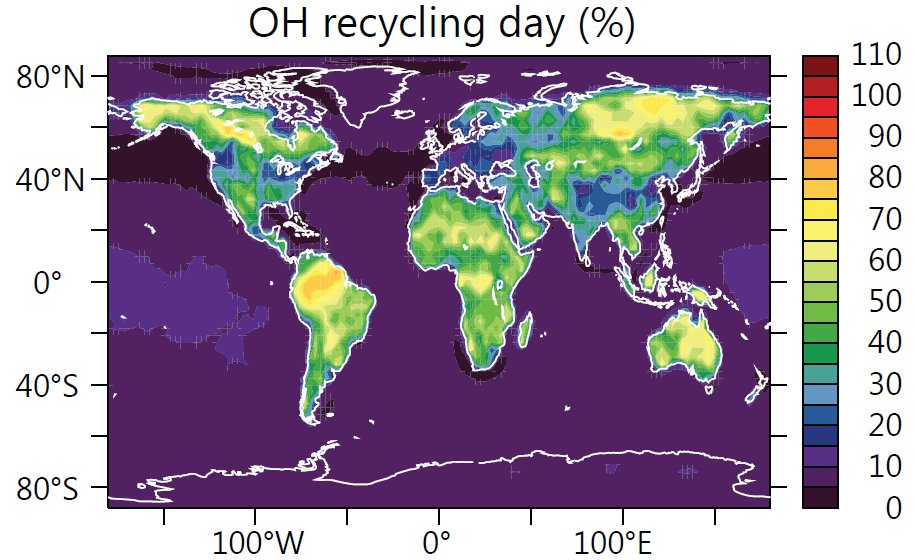
Measurements of the OH radical above the tropical rain forests suggest that OH radicals are efficiently recycled after reacting with isoprene. Isoprene is a natural plant gas that is emitted into the atmosphere in large quantities. It is estimated that plants annually produce more than 400 million tons of the hydrocarbon with the majority produced during the day in tropical rain forests.
So far it was unclear how the chemical recycling works in details. Additionally, models of atmospheric chemistry and OH measurements above the tropical rain forests do not match. Scientists thought that isoprene contributes only to the reduction of OH radicals.
"In our opinion, the system is more complex, because the oxidation of isoprene contributes to the reduction as well as to the formation of OH radicals, so that it sustains and limits itself," said Domenico Taraborrelli, lead author of a new study. "The recycling efficiency of OH radicals thus depends on their quantity. If the OH concentration is high, little is recycled, if it is low, a lot is produced," adds the atmospheric chemists.
In order to better reconcile theory and measurements, the scientists advanced a model of global atmospheric chemistry with complex reaction cascades. Accordingly, isoprene is converted by OH radicals into unsaturated hydroperoxy aldehydes. At low OH radical concentration sunlight leads to a net production of OH radicals due to the further oxidized isoprene degradation products. If however the OH concentration is high, the radical is consumed by the oxidation of the degradation products.
For the interaction of biosphere and atmosphere, the buffering of the OH radical quantity is very important, since large forests can maintain their self-cleaning capacity.
"Our results also indicate that increasing isoprene emissions, as we shall see them through global warming, will not enhance the climate effect," concludes Jos Lelieveld, director at the Max Planck Institute for Chemistry. "This shows that natural ecosystems are often better buffered than we suspected.”
Next, the Mainz researchers aim at investigating how stable is the atmospheric oxidation capacity against perturbations like sudden methane releases from permafrost soils. Through global warming great areas for example in Russia thaw up and release huge amounts of methane gas.