Mass Spectrometry – More Than a Measurement Method
Station 6

Until the 1970s, mass spectrometry was more than just a method for scientific problem-solving at the MPI for Chemistry. It bridged the gap between nuclear chemistry and nuclear physics, once the Institute’s key areas of research.
The underlying principle involves accelerating positively or negatively charged ions of a sample material and then deflecting them with electrical or magnetic fields in order to determine mass and frequency of atoms. This made it possible, for example, to show that elements consist of several isotopes – atomic variants having the same number of protons but different numbers of neutrons in the nucleus. This means that they differ in mass but not in chemical behaviour.
This realization allowed many questions in nuclear physics to be answered relatively easily. The work was continued later in the departments of geochemistry and cosmochemistry, where mass spectrometry was used to assist in the analysis of terrestrial and extraterrestrial rocks. This, together with particle accelerators, allowed greater particle measurement accuracy to be achieved, which is why mass spectrometry also became an important tool in nuclear physics. It can be used to measure nuclear binding energies, which sheds light on the structure of atomic nuclei and the sequence of nuclear reactions.
Mass spectrometry continues to play an im-portant role at the MPI for Chemistry today. For instance, it is used in the chemical analysis of aerosol particles, fossils and stalagmites.
(partly quoted: C. Reinhardt, Festschrift MPIC 2012)
Multi-Ion Counting System
Exhibit 6a+b
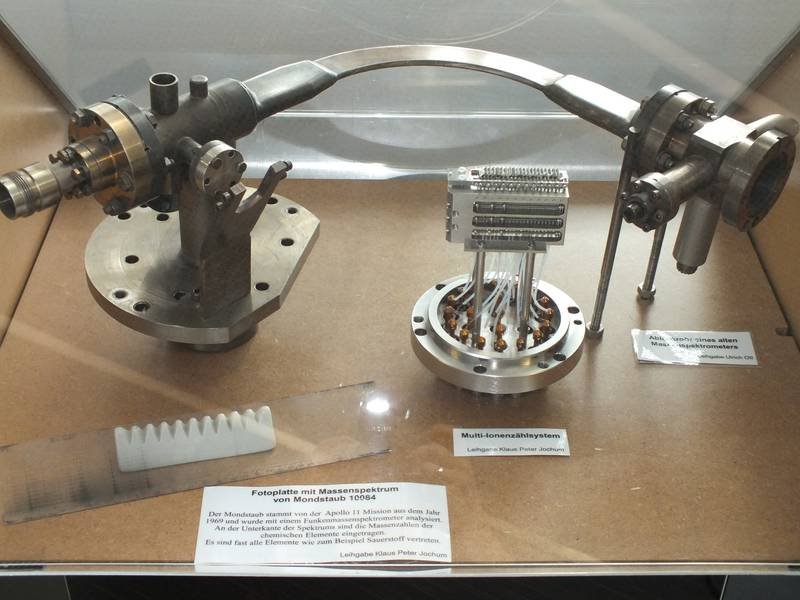
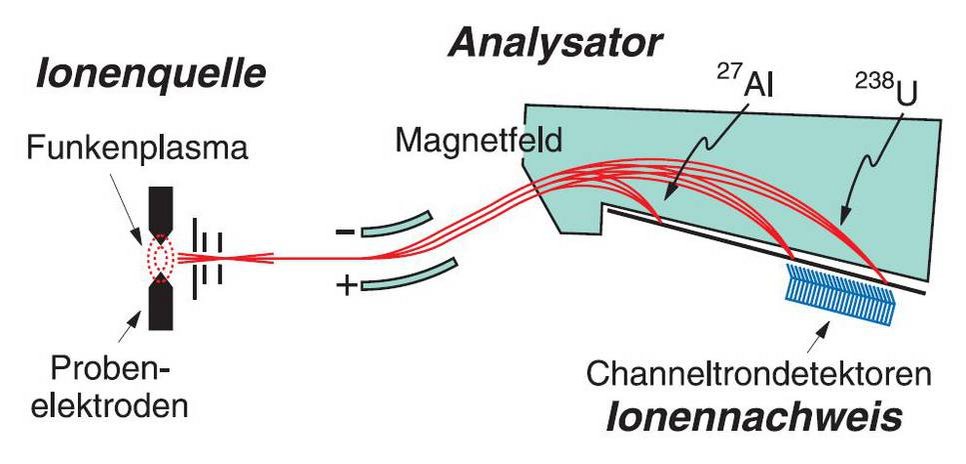
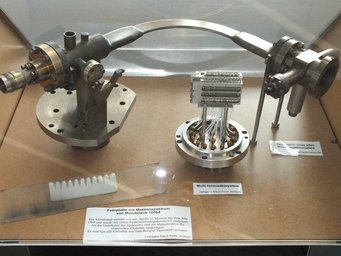
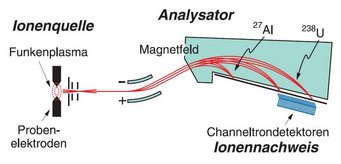
The device is a multi-ion counting system from a former spark mass spectrometer. It could detect up to 20 different chemical elements simultaneously, for example from rock samples. The system is a proprietary development by the MPI for Chemistry and was built in the workshop in 1995. It replaced the less accurate photographic plates, in which ions darkened a photographic plate.
On the bottom of the apparatus you can see 20 diagonally arranged metal plates. When high-energy ions hit these plates, they released electrons. The electrons were then directed into extremely thin tubes called channeltrons and multiplied in an avalanche-like fashion.
Pulse counters at the end of the tubes registered the number of electrons, which determined the amount of each chemical element.
The detection system was preceded by an ion source and an analyzer. In the ion source, high-frequency voltage heated the rock sample to the point where plasma – a hot gas of ionized atoms – was formed. A magnetic field then separated the ions according to their mass.
This means that elements with large atomic masses – such as uranium-238 or thorium-232 – were deflected much less than elements with small atomic masses, such as aluminum-27. As a result, they hit different metal plates and could be separated.
Modern mass spectrometers today use the same principle.