Soil, Plants, Air – the Earth Sciences

Station 12
If you were to define the Earth as a system, it consists of the atmosphere, the biosphere, the hydrosphere, the geosphere and the impact of humans, termed the anthroposphere. Changing any one of these factors could have an impact on the system as a whole, since feedback effects and remote global effects are integral parts of the system. The discipline that deals with almost all aspects of the Earth system is called biogeochemistry.
The idea to base this field of research at the MPI for Chemistry emerged in the 1970s from diverse scientific investigations into atmospheric chemistry. At the time, colleagues of the meteorologist and atmospheric chemist Christian Junge – Director of the MPI for Chemistry from 1968 − were already conducting research into the biogenic formation of trace gases such as carbon monoxide and methane. Junge’s successor, Paul Crutzen also undertook many projects linked to biogeochemistry. The Biogeochemistry Department was essentially established on his initiative. Meinrat O. Andreae was appointed director in 1986.
Interestingly, this relatively new line of research touches on many of the themes of the earlier KWI for Chemistry. Not only photosynthesis experiments by Willstätter, but also research into carbonyl sulphide by Alfred Stock would now fall under the field of biogeochemistry.
Marine Plankton, Sulfur, and Climate
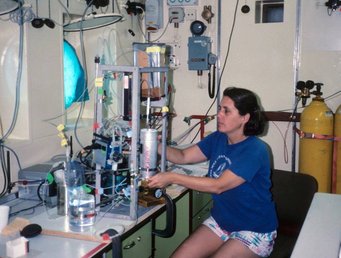
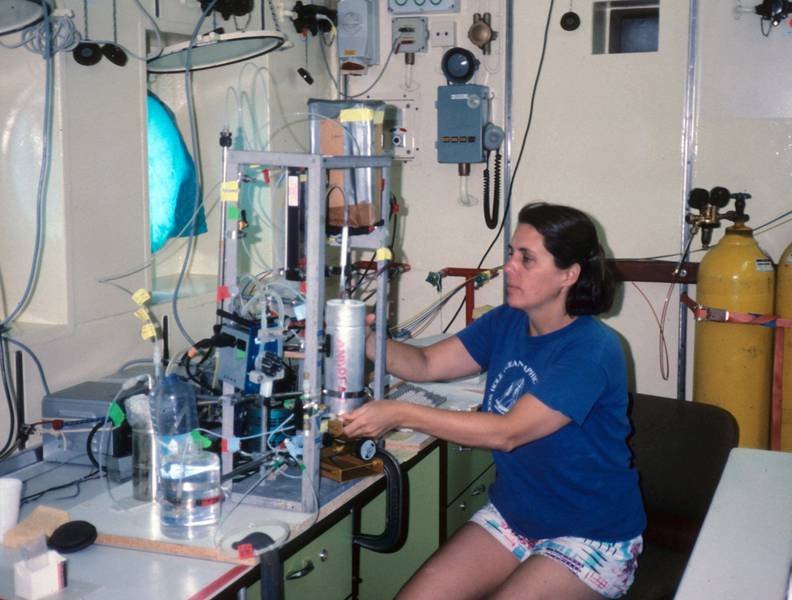
Exhibit 12: Andreae’s strain gauge measuring device
Dimethylsulfide (DMS) is a critical link between the biosphere and climate. This sulfur compound is produced by phytoplankton in the oceans and escapes from seawater into the air. In the atmosphere, sunlight oxidizes it into sulfate particles. These particles in turn act as cloud condensation nuclei. In many remote marine regions, DMS is the primary source of condensation nuclei.
To determine where and how much DMS is produced, Meinrat O. Andreae built a simple and portable instrument in the 1980s that could measure DMS in both seawater and air.

Air samples were passed through quartz glass tubes lined with gold wool to concentrate DMS. Seawater samples were depleted of DMS by bubbling them with helium in a chamber and then drying the gas. The samples were then passed into a U-shaped tube containing a substance that traps DMS. The tube was immersed in liquid nitrogen to fix the sample.
Analysis was performed using a gas chromatograph, and the U-tube was heated prior to analysis.
The instrument was in use worldwide from 1987 to 1991, measuring DMS concentrations directly on board research vessels such as the MS Meteor.